Supplement packaging is the combination of science, test, and clever design. No matter what form your products are in, reliable packaging is the protection of the reputation of your brand and your products as well.
In this blog, Meishida, a leading pharmaceutical packaging manufacturer, will explain that Innovative packaging solutions are becoming a strategic tool — not just a protective layer. From advanced barrier films to intelligent moisture-control structures, modern supplement packaging now plays an active role in preserving potency, shelf life, and brand credibility.
The Main Threats to Supplement Stability
Supplements face predictable hazards in storage and transport.
- Oxygen drives oxidative decay of vitamins and botanical actives.
- Moisture causes clumping in powders and sticky gummies.
- Light and UV radiation degrade photosensitive compounds.
- Temperature swings accelerate chemical reactions and can compromise texture or microbial safety.
Understanding which of these threats matters most for your formula is the first step in specifying effective supplement packaging.
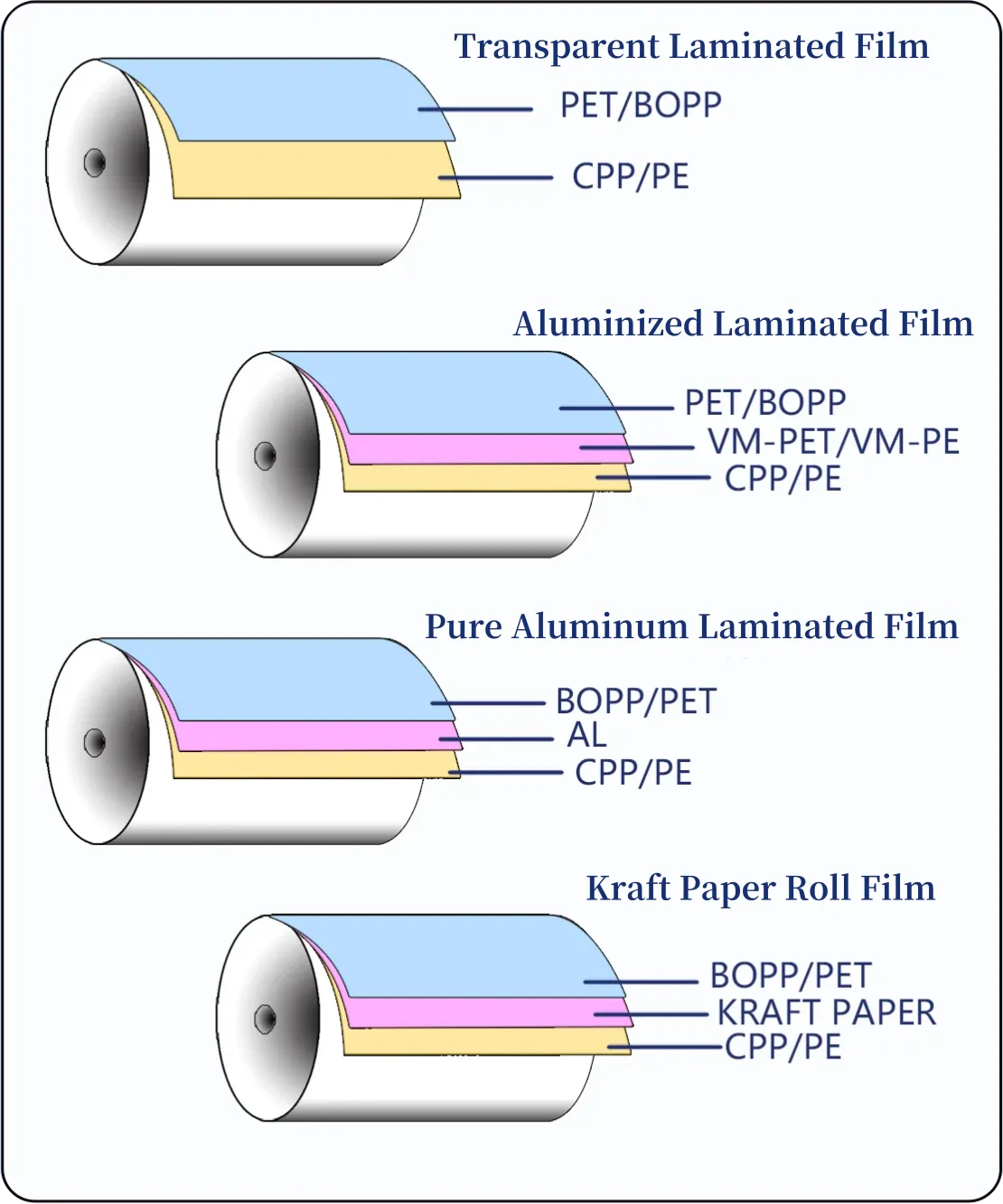
Passive High-Barrier Materials — the Baseline
High-barrier materials form the foundation of long shelf life. Common solutions include:
- Aluminum foil laminates (PET/AL/PE): excellent light and oxygen barrier; widely used for long-life products and export SKUs.
- EVOH-containing laminates: deliver low oxygen transmission while allowing transparent or semi-transparent finishes for marketing impact.
- Glass and lined HDPE/PP bottles: inert, durable, and well-suited to tablets and capsules where repeated opening occurs.
- Mono-material pouches (PP/PP, PE/PE): emerging as recyclable options where barrier requirements are moderate.
For each material, set target OTR (oxygen transmission rate) and MVTR (moisture vapor transmission rate) values that align with the formula’s sensitivity and your intended shelf life.
Active Packaging Technologies — Controlling the Microclimate
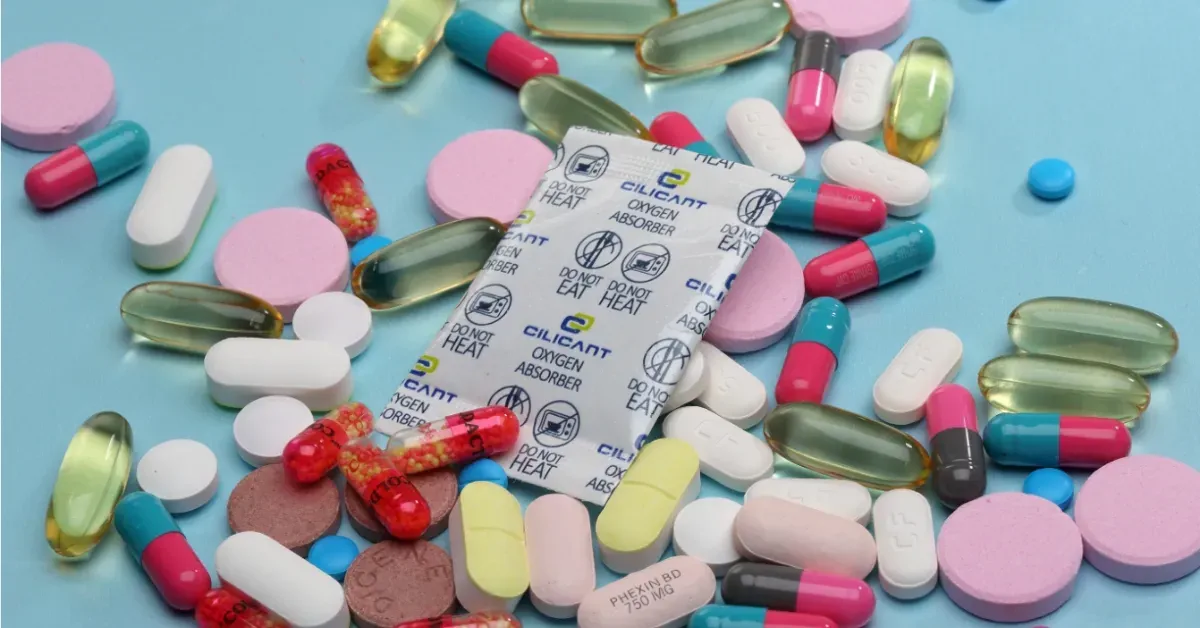
Passive barriers can be combined with active elements to control the internal atmosphere:
- Oxygen absorbers reduce headspace oxygen quickly and reliably, ideal for powders and oxygen-sensitive vitamins.
- Desiccants and humidity regulators prevent moisture uptake in hygroscopic powders and gummy products.
- Combined O₂ + desiccant sachets are useful when both threats are present.
- Modified atmosphere packaging (MAP) and nitrogen flushing are used during fill to displace oxygen before sealing.
- Antimicrobial liners or coatings can raise microbial safety for certain liquid or wet-fill formulations — but these require validated claims and regulatory checks.
Active systems are cost-effective for high-value products where potency loss translates to revenue loss or safety risk.
Structural Formats Matched to the Dose Form
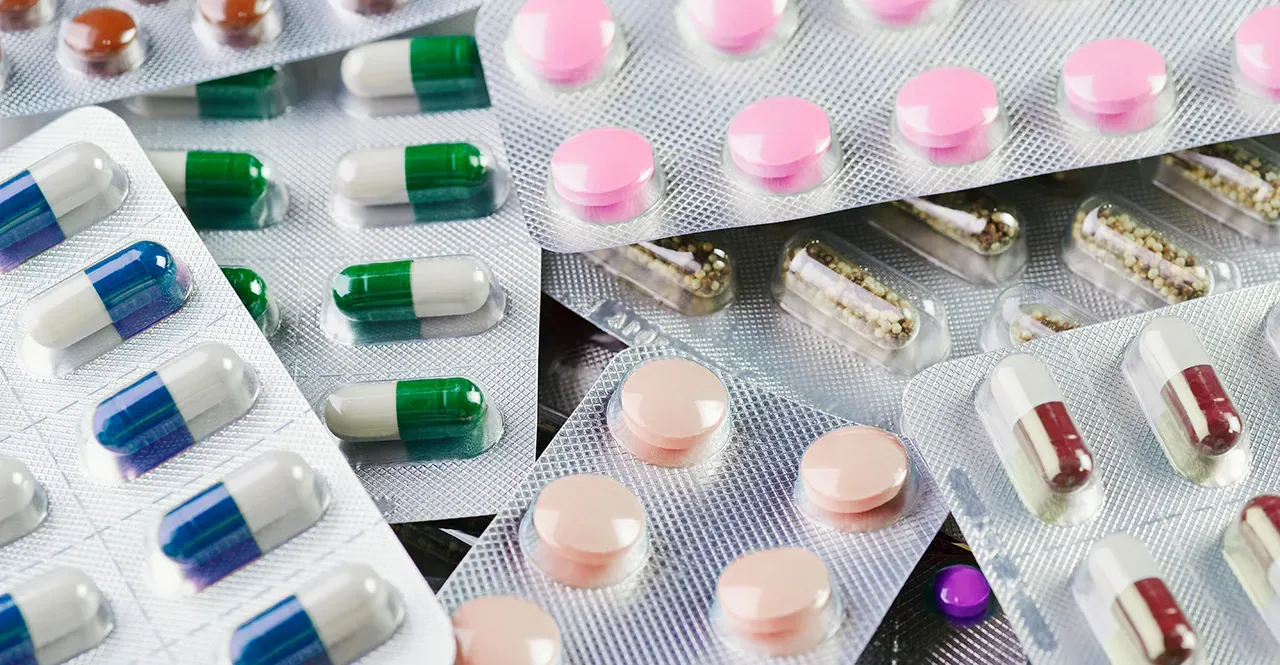
The packaging format itself influences exposure risk and user convenience:
- Blister packs (Alu-Alu / Alu-PVC): top choice for single-dose tablets and moisture-sensitive formulations. Alu-Alu offers the highest barrier and long-term stability.
- Stick packs & single-serve sachets: minimize repeated exposure and are excellent for probiotics, pre-workout powders, and travel-friendly dosing.
- Stand-up pouches with resealable zippers: work well for bulk powders and snack-like supplements, especially when combined with oxygen absorbers or valve systems.
- Spout pouches: ideal for liquid concentrates and oil-based botanicals; require high-barrier films and secure tamper-evident closures.
- Bottles with desiccant-lined caps: common for tablets and capsules where repeated access is required.
Choose the format that matches dosing behavior: single-serve for daily disposable dosing; resealable bulk for repeated dosing with effective active controls inside.
Smart Packaging and Monitoring
Intelligent indicators can reduce risk and improve traceability:
- Time–temperature indicators (TTIs) show whether the product has been exposed to damaging heat during transit.
- Oxygen or humidity indicator cards provide visual assurance of package integrity.
- QR/NFC-enabled traceability links consumers to batch COAs, storage instructions, and freshness guidance.
- Cold-chain loggers are useful for temperature-sensitive liquid or probiotic products.
Smart elements help brands manage recalls, validate distribution, and maintain consumer trust. By investing in innovative supplement packaging solutions, brands can significantly reduce product degradation while strengthening consumer trust and long-term market positioning.
Sustainability Trade-Offs and Practical LCA Thinking
There’s a tension between high barrier performance and recyclability. Practical approaches include:
- Design-for-recycling using mono-material pouches where feasible (PP/PP, PE/PE) and balancing barrier needs with shelf-life.
- Lightweighting to reduce embodied carbon while maintaining barrier efficacy.
- PCR content in non-food-contact layers and closures.
- Take-back and refill models for bulk products to reduce single-use waste.
Evaluate packaging decisions via functional LCA: measure CO₂ per dose preserved, not just per unit of material.
Quick Decision Matrix
- Identify the most damaging factor for your SKU (oxygen/moisture/light/heat).
- Set your target shelf life and distribution profile.
- Choose a base material that meets the required OTR/MVTR numbers.
- Add active elements (oxygen absorbers, desiccants) where passive barriers alone are insufficient.
- Select the format that suits dosing behavior and minimizes exposure.
- Validate under accelerated and real-life distribution conditions.
- Incorporate sustainability trade-offs into the LCA and choose recyclable options where possible.
Conclusion
Effective supplement packaging combines robust barrier materials, active microclimate control, appropriate format design, and rigorous testing. The right combination preserves potency, protects consumers, and reduces commercial risk.
明石田について

Meishida is a leading pharmaceutical packaging manufacturer, specializing in offering wholesale supplement packaging supply and custom supplement packaging solutions for global brands. We operate with internationally recognized safety standards and maintain rigorous production control to ensure consistent quality.
As a factory-direct partner in the supplement packaging industry, we deliver reliable materials, stable lead times, and packaging solutions tailored to real market needs.
Partner with Meishida, and you are provided:
✅Excellent quality
✅One-stop custom service
✅BRC, ISO, FDA certified products
✅Innovative technology support
✅Environmentally friendly philosophy
✅Quick expert team response





