Meishida has to mention that a poorly specified pouch can cause clumping, oxidation, off-flavor, or recalls; a well-specified one protects actives and reduces logistics cost. It is rather instrumental for the procurement team to consider when sourcing supplement packaging.
As a pharmaceutical packaging manufacturer, we bring you a blog that covers the practical material, process, testing, and commercial issues you should insist on when sourcing a supplement pouch.
Start with the Product: Format Drives Requirements
Different supplement formats impose distinct supplement packaging demands:
- Powders (protein, greens, collagen) — primary concerns are moisture ingress, caking, and static; robust moisture barrier and anti-caking strategies are required.
- Capsules/tablets — oxygen sensitivity and abrasion protection are key; innerliners, desiccants, and rigid/laminated structures are common.
- Gummies/chewables — moisture and stickiness control; high MVTR resistance and choice of inner coatings to reduce migration.
- Liquids/serums — leak prevention and chemical compatibility with sealants and closures.
Define the product’s critical quality attributes (CQAs) up front: target shelf life, acceptable nutrient retention (e.g., % active retained at 6 months), and distribution profile (cold chain vs ambient, domestic vs export).
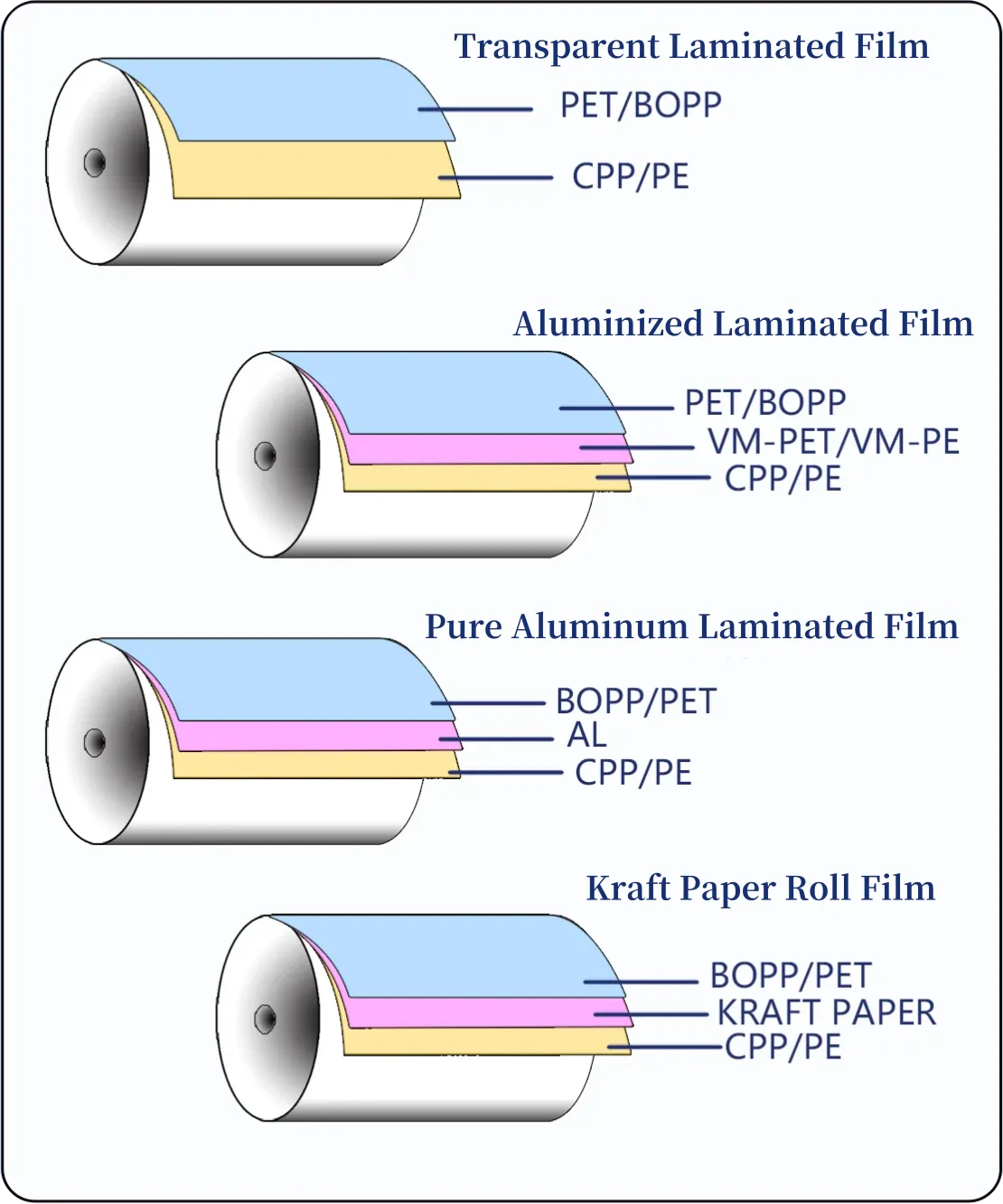
Material Structures — Match Barrier to Biology
Supplement pouches are laminated. Each layer performs a function: printability, barrier (oxygen, moisture, light), mechanical strength, and a sealant for heat sealing or spout welding.
Common structures and when to use them:
- PET/AL/PE (foil laminate) — maximum light and oxygen barrier; preferred for long ambient shelf life or highly oxidation-sensitive actives.
- PET/EVOH/PE — high oxygen barrier with partial transparency; suitable when some product visibility is desired but oxygen control is still important.
- PET/NY/PE or PA/PE — adds puncture resistance for rugged logistics or particulate products.
- PE/PE or PP/PP mono-material — improves recyclability; consider only for local distribution and shorter shelf life, or pair with oxygen absorbers.
Set measurable targets: typical buyers specify OTR and MVTR (for example, target OTR <1–5 cc/m²·day for highly oxygen-sensitive blends; MVTR <0.5 g/m²·day where moisture control is critical). Your lab or CPO should confirm realistic targets based on the intended shelf life.
Closures and Reseal Systems — Functional Risk Areas
Zippers, sliders, and spouts create user value but also potential failure points.
- Zipper performance: requires cycle testing (e.g., 50–200 open/close cycles) and residual leak tests.
- Child-resistant closures: if required for regulated products, verify compliance and usability testing.
- Spouts: specify material (PP for hot processes, PE for ambient), inner liners, torque retention, and leak thresholds.
- Tamper evidence: define acceptable tamper features and test for integrity after transport simulation.
Insist on cap/spout DoC and torque retention data under temperature variation.
Filling, Process Compatibility, and In-line Controls
How you fill determines permissible supplement pouch structures.
- Hot-fill is forgiving microbiologically but lowers labile nutrients; confirm laminate heat tolerance and sealant compatibility.
- Aseptic offers the best nutrient retention but requires certified sterile film and validated processes.
- Nitrogen blanketing/vacuum filling lowers initial headspace O₂ — specify target residual O₂ (e.g., <1%) and require headspace O₂ measurement data.
- Powder fillers: verify anti-caking strategies, dust control, and electrostatic mitigation; pouch closure must maintain seal integrity even with particulate ingress.
Request a witnessed pilot run or line trial on your intended filler — lab data alone is insufficient.
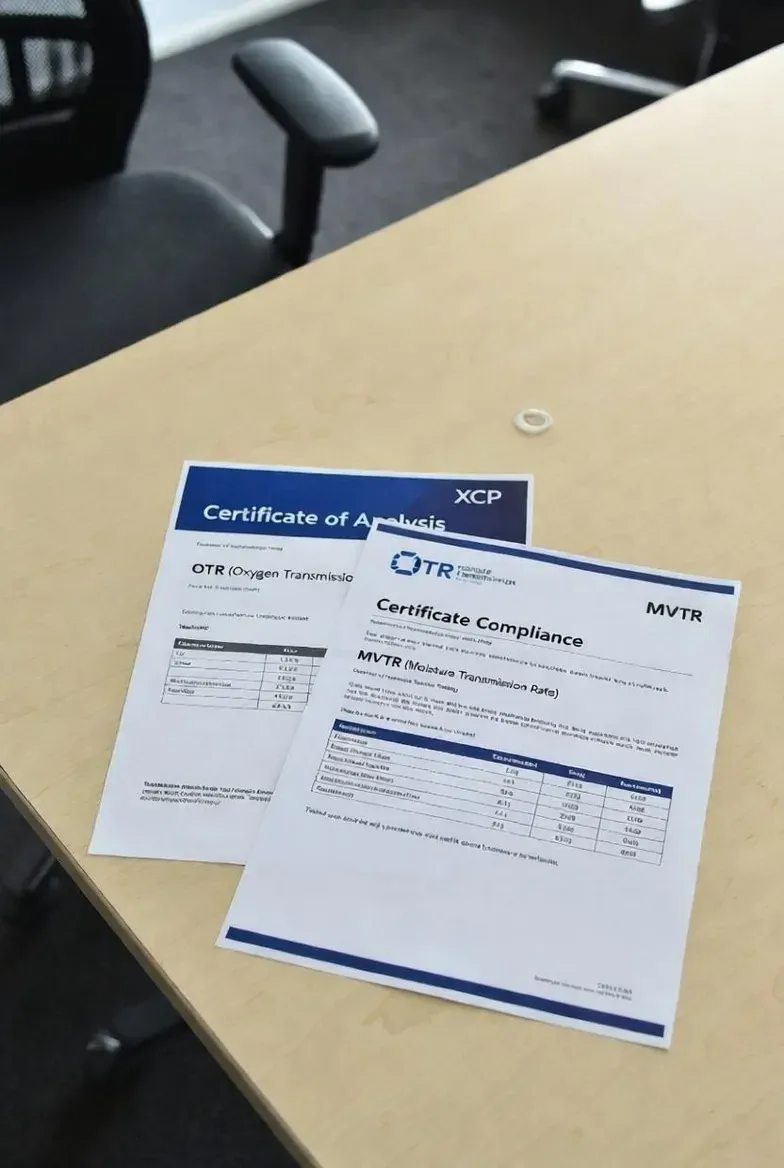
Mandatory Testing and Documentation
Do not accept bulk supply without the following documentation and reports:
- Material spec & Declaration of Compliance (DoC) for every film layer, inks, adhesives, spout, and zipper.
- Migration testing (overall and specific) using appropriate food simulants; supply test reports for worst-case temperature and pH.
- Organoleptic (odor/taste) tests after accelerated storage.
- OTR / MVTR certificates for laminate under actual laminate thickness.
- Seal strength, burst, and leak tests (with spout/zippers installed). Specify test methods and acceptance criteria (e.g., seal peel N/15mm).
- Shelf-life validation: accelerated (40°C/75%RH) and real-time, with nutrient retention metrics where applicable (e.g., percent vitamin retained).
- GMP / cGMP evidence for the converter; for U.S. supplements, reference FDA 21 CFR Part 111 expectations for packaging suppliers.
- Lot traceability & QC plan: film lot, spout lot, production batch record, and recall procedures.
Require these documents as contractual deliverables before purchase orders are released.
Active Measures and Design Choices
For borderline distribution or highly sensitive actives, consider active interventions:
- Oxygen absorbers inside the pouch for powders or multi-serve formats.
- Desiccant sachets or moisture-buffering liners for hygroscopic blends.
- Barrier coatings or SiOx/AlOx as alternatives to metallization when metal detection or recyclability is a concern.
Document how these devices are integrated, their shelf life, and safety data.
Commercial Terms, Risk Allocation, and Supply Chain Practice
Practical procurement points:
- MOQ & lead time: Gravure printing and lamination have higher setup costs; digital short runs reduce MOQ but increase unit cost.
- Tooling ownership: clarify whether printing cylinders, spout tooling, or molds are customer or supplier property.
- Material substitution: forbid unapproved material substitutions in the contract, or require 30-day advance notice and re-qualification.
- Warranty & shelf-life liability: include acceptance testing and liability clauses tied to agreed shelf-life KPIs.
- Change management: define the process for ink, adhesive, or film supplier changes and required retesting.
Negotiate contract items that tie supplier liability to predefined functional acceptance criteria, not vague statements, when sourcing supplement pouches.
Actionable Short Checklist
- Define CQAs: shelf life, target % active retention, distribution profile.
- Request laminate spec, OTR/MVTR, and DoC for layers and closures.
- Receive migration & organoleptic reports for finished laminate.
- Require a pilot-run sample on your filler with seal, burst, and leak tests.
- Confirm GMP evidence and QC sampling plan.
- Contractually fix material substitution, MOQ, lead time, and shelf-life liability.
Conclusion
Treat the supplement pouch as a product quality control, not just packaging. When procurement teams define technical acceptance criteria, enforce pilot validation, and secure the necessary documentation, the pouch becomes a reliable part of your product quality system — protecting actives, reducing risk, and enabling predictable commercial outcomes.
À propos de Meishida

At Meishida, we provide wholesale supplement pouch service and custom supplement packaging solutions. We operate advanced 100,000-grade purification workshops and a 10,000-grade laboratory, supported by ISO9001 and ISO14001 certifications, ensuring high manufacturing standards and product safety.
For two decades and more, Meishida has earned strong recognition for quality, precision, and reliability in customized packaging from our global clients.
FAQ: Common Procurement Mistakes to Avoid When Sourcing a Supplement Pouch
Q1: Is it a mistake to choose a supplement pouch mainly based on appearance?
Yes. Visual design matters for branding, but selecting a supplement pouch primarily for its look often leads to insufficient oxygen or moisture protection. Barrier specifications such as OTR and MVTR should always come before aesthetics, especially for supplements sensitive to oxidation or humidity.
Q2: Why is skipping a filling-line pilot risky for a supplement pouch?
A supplement pouch that performs well in lab tests may fail during real filling. Without a pilot run on the actual filling line, issues such as poor sealing, powder contamination, or spout leakage can remain hidden until mass production.
Q3: Are generic Declarations of Compliance acceptable for a supplement pouch?
No. A supplement pouch requires layer-specific Certificates of Analysis and Declarations of Compliance for films, inks, adhesives, and closures. Generic documentation increases regulatory and recall risk.
Q4: Why does traceability matter for supplement pouch procurement?
End-to-end traceability ensures each supplement pouch can be linked to specific film and spout lots. Without it, quality investigations and recalls become costly and slow.
Q5: Is the lowest unit price a good strategy for buying a supplement pouch?
Not usually. Focusing only on unit price ignores total cost, including waste, returns, and recall exposure. A well-specified supplement pouch often reduces long-term operational risk and overall cost.

.webp)





